Graphene: The Role of Carbon (6 Protons, 6 Neutrons, 6 Electrons)
The story of graphene begins not with grand pronouncements or cutting-edge technology, but with the quiet elegance of an atom – carbon. Carbon, element number 6 on the periodic table, is a fundamental building block of life, a versatile architect of molecules, and, in this particular tale, the key to unlocking the extraordinary properties of graphene.
Deep within the heart of every carbon atom resides a nucleus, a tightly packed core of 6 protons and 6 neutrons. These particles, bound together by the strong nuclear force, define the very essence of carbon. Circling this nucleus, like a miniature solar system, are 6 electrons. These electrons, governed by the laws of quantum mechanics, occupy specific energy levels and orbitals, their dance dictating how carbon interacts with other elements, how it forms bonds, how it builds structures both complex and simple.
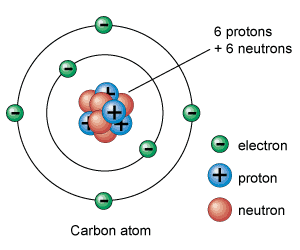
This dance of 6 – 6 protons, 6 neutrons, 6 electrons – is not merely a numerical curiosity. It’s the foundation upon which carbon’s remarkable versatility rests. It allows carbon to form four covalent bonds, a flexibility that makes it the backbone of organic chemistry and the basis of all known life. But it’s also this dance that gives rise to graphene, a material so thin it’s practically two-dimensional, yet so strong it rivals diamond.
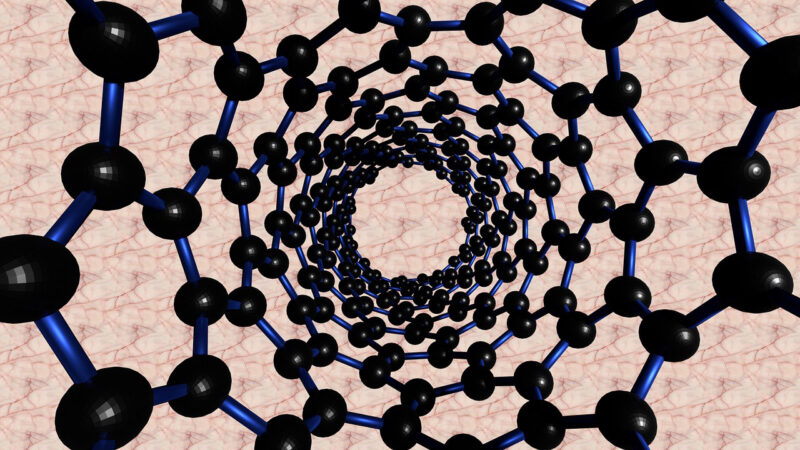
Imagine taking a sheet of graphite, the stuff of pencil lead, and peeling away layer after layer. Eventually, you arrive at a single, solitary sheet, just one atom thick. This is graphene. It’s a honeycomb lattice of carbon atoms, each one connected to three neighbors in a perfect hexagonal pattern. And it’s this pattern, this specific arrangement born from the dance of 6, that gives graphene its extraordinary properties.
The carbon atoms in graphene aren’t just connected; they’re locked in an embrace of shared electrons. These electrons, no longer confined to individual atoms, roam freely across the entire sheet, creating a sea of conductivity. This is why graphene conducts electricity so efficiently, far surpassing even copper in certain applications.
But graphene is more than just a superb conductor. The tight, strong bonds between the carbon atoms, forged by the dance of 6, make it incredibly strong, hundreds of times stronger than steel. And because it’s only one atom thick, it’s also remarkably light and transparent.
The journey from the carbon atom to graphene is a journey of scale, from the infinitesimal world of protons, neutrons, and electrons to the macroscopic world of materials and applications. It’s a journey of organization, where the simple dance of 6 gives rise to complex structures and emergent properties.
Scientists are still unraveling the full potential of graphene. They envision it revolutionizing electronics, creating flexible displays, faster transistors, and more efficient solar cells. They see it strengthening materials, building lighter and more durable structures. They even imagine it playing a role in medicine, delivering drugs, sensing biomolecules, and even regenerating tissues.
The story of graphene is far from over. It’s a story that continues to unfold as researchers explore its properties and discover new ways to harness its potential. But it’s a story that begins with the fundamental dance of 6, the dance of carbon’s protons, neutrons, and electrons, a dance that has shaped life itself and now promises to reshape the world around us.